The concept of energy units is fundamental to understanding various physical and chemical phenomena. Energy, a measure of the ability to do work, is quantified in different units depending on the context and system of measurement being used. One of the primary energy units is the Joule (J), which is defined as the energy expended when a force of 1 Newton is applied over a distance of 1 meter. However, for smaller scales, such as in chemistry and atomic physics, other units like the electronvolt (eV) are more commonly used due to their relevance to the energy levels of electrons in atoms and molecules.
Key Points
- The Joule (J) is a fundamental unit of energy in the International System of Units (SI), used to measure energy in various forms such as mechanical, thermal, and electrical.
- The electronvolt (eV) is a unit of energy particularly useful in atomic and subatomic physics, representing the energy gained by an electron when accelerated through a potential difference of 1 volt.
- Calorie (cal) is a unit of energy often used in nutrition to measure the energy content of foods, though it is not part of the SI system.
- British Thermal Units (BTU) are used primarily in the United States to measure the energy content of fuels and the efficiency of heating and cooling systems.
- Kilowatt-hours (kWh) are a unit of energy used to measure electrical energy, particularly in the context of electrical power generation and consumption.
Understanding Energy Units
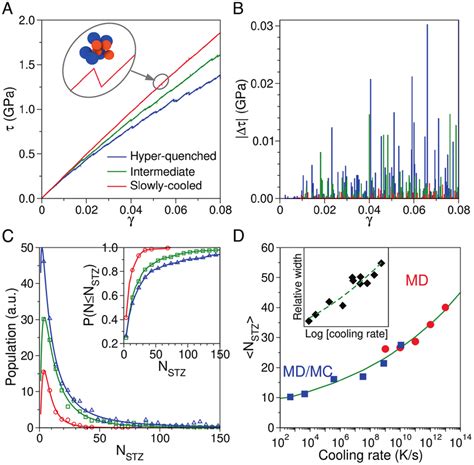
Energy units are crucial for quantifying and comparing different forms and amounts of energy. The choice of unit depends on the specific application and the magnitude of the energy being considered. For instance, the energy released by a chemical reaction might be expressed in Joules, while the energy consumption of a household appliance is often given in kilowatt-hours (kWh). The conversion between different energy units is straightforward, with conversion factors derived from the definitions of the units. For example, 1 kWh equals 3.6 million Joules (since 1 kWh = 1000 watts * 3600 seconds, and 1 watt = 1 Joule/second).
Electronvolts (eV) in Atomic Physics
In the realm of atomic physics, the electronvolt (eV) is a particularly useful unit of energy. It is defined as the energy gained by a single electron when it moves through an electric potential difference of 1 volt. This unit is convenient for describing the energy levels of electrons in atoms and molecules, as well as the energies associated with chemical bonds and reactions. For example, the energy required to remove an electron from a hydrogen atom (the ionization energy) is approximately 13.6 eV.
| Energy Unit | Definition | Common Use |
|---|---|---|
| Joule (J) | 1 N*m | General energy measurements |
| Electronvolt (eV) | Energy gained by an electron moving through 1 V | Atomic and subatomic physics |
| Calorie (cal) | Approximately 4.184 J, originally defined as the energy needed to raise the temperature of 1 gram of water by 1°C | Nutrition and food science |
| British Thermal Unit (BTU) | Approximately 1055 J, originally defined as the energy needed to raise the temperature of 1 pound of water by 1°F | Energy content of fuels, heating and cooling systems |
| Kilowatt-hour (kWh) | 3,600,000 J, or the energy consumed by a 1 kW device in 1 hour | Electrical energy consumption and production |
Applications and Conversions
Understanding the relationships and conversions between different energy units is essential for applying them correctly in various contexts. For example, in environmental science, comparing the energy efficiency of different fuels or technologies requires converting between units such as Joules, BTUs, and kWh. Similarly, in nutritional science, converting between calories and Joules helps in understanding the energy content of foods and the energy expenditure of the human body.
Practical Considerations
In practical applications, the selection of an energy unit can simplify complex calculations and facilitate communication among professionals. For instance, electrical engineers prefer kWh for discussing power generation and consumption because it directly relates to the operation of electrical systems. In contrast, chemists might use Joules or calories when discussing the energy changes in chemical reactions, as these units are more directly related to the molecular scale.
What is the most commonly used energy unit in physics?
+The Joule (J) is the most commonly used energy unit in physics, as it is part of the International System of Units (SI) and can be applied to various forms of energy, including mechanical, thermal, and electrical.
How do you convert between different energy units?
+Conversions between different energy units are performed using conversion factors derived from the definitions of the units. For example, to convert kWh to Joules, you multiply by 3,600,000 (since 1 kWh = 3.6 million Joules).
What is the significance of electronvolts (eV) in atomic physics?
+Electronvolts (eV) are significant in atomic physics because they provide a convenient unit for describing the energy levels of electrons in atoms and molecules, as well as the energies associated with chemical bonds and reactions.
In conclusion, energy units play a vital role in quantifying and comparing different forms and amounts of energy across various scientific and engineering disciplines. Understanding the definitions, applications, and conversions between these units is essential for effective communication and calculation in energy-related contexts. By recognizing the appropriateness of each unit for specific applications, professionals can ensure clarity, precision, and relevance in their work, whether it involves the design of more efficient electrical systems, the development of new materials, or the assessment of nutritional content in food products.