The calculation of the molar mass of MgOH2, also known as magnesium hydroxide, involves determining the sum of the atomic masses of its constituent elements. Magnesium hydroxide is composed of one magnesium (Mg) atom, two hydrogen (H) atoms, and two oxygen (O) atoms. To calculate its molar mass, we need to know the atomic masses of these elements.
The atomic masses of the elements in MgOH2 are approximately: magnesium (Mg) = 24.305 g/mol, hydrogen (H) = 1.008 g/mol, and oxygen (O) = 15.999 g/mol. Using these values, we can calculate the molar mass of MgOH2 as follows: Molar mass of MgOH2 = (1 * atomic mass of Mg) + (2 * atomic mass of H) + (2 * atomic mass of O).
Calculation Process

To proceed with the calculation: Molar mass of MgOH2 = (1 * 24.305 g/mol) + (2 * 1.008 g/mol) + (2 * 15.999 g/mol). Performing the arithmetic: Molar mass of MgOH2 = 24.305 g/mol + 2.016 g/mol + 31.998 g/mol.
Final Calculation
Adding these values together: Molar mass of MgOH2 = 24.305 g/mol + 2.016 g/mol + 31.998 g/mol = 58.319 g/mol. Therefore, the molar mass of MgOH2 is approximately 58.319 grams per mole.
| Element | Atomic Mass (g/mol) | Number of Atoms in MgOH2 | Total Mass Contribution (g/mol) |
|---|---|---|---|
| Magnesium (Mg) | 24.305 | 1 | 24.305 |
| Hydrogen (H) | 1.008 | 2 | 2.016 |
| Oxygen (O) | 15.999 | 2 | 31.998 |
| Total | 58.319 |
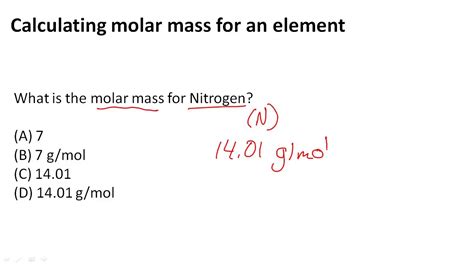
Key Points
- The molar mass of MgOH2 is calculated by summing the atomic masses of its constituent elements: magnesium, hydrogen, and oxygen.
- The atomic masses used are approximately 24.305 g/mol for magnesium, 1.008 g/mol for hydrogen, and 15.999 g/mol for oxygen.
- The calculation involves multiplying the atomic mass of each element by the number of atoms of that element in the compound and then summing these values.
- The molar mass of MgOH2 is approximately 58.319 g/mol, which is essential for quantitative chemical analyses and reactions involving this compound.
- Understanding molar mass calculations is crucial for chemists and researchers to accurately prepare solutions, predict reaction outcomes, and analyze the composition of substances.
Applications of Molar Mass Calculations

Molar mass calculations have numerous applications in chemistry and related fields. They are used in the preparation of solutions with specific concentrations, in the calculation of the amounts of reactants needed for chemical reactions, and in the analysis of the composition of unknown substances. For MgOH2, knowing its molar mass is essential in pharmaceutical applications, as it is used as an antacid and a laxative, and its dosage must be precisely controlled.
Pharmaceutical Applications
In pharmaceutical formulations, the molar mass of MgOH2 is critical for determining the correct dosage. Since MgOH2 is used to neutralize stomach acid and as a laxative, its concentration in a formulation must be carefully controlled to ensure efficacy and safety. The molar mass allows pharmacists and manufacturers to accurately calculate the amount of MgOH2 needed per dose, ensuring that the product is both effective and safe for consumption.
The calculation of the molar mass of compounds like MgOH2 underscores the importance of fundamental principles in chemistry. By understanding and applying these principles, chemists and researchers can develop new materials, design more efficient chemical processes, and ensure the quality and safety of products that affect our daily lives.
What is the significance of calculating the molar mass of a compound like MgOH2?
+The calculation of the molar mass of MgOH2 is significant because it allows for the precise quantification of the substance in various applications, including chemical reactions, solution preparation, and pharmaceutical formulations.
How does the molar mass of MgOH2 impact its use in pharmaceutical applications?
+The molar mass of MgOH2 is crucial in pharmaceutical applications for determining the correct dosage of the compound. It ensures that the formulation is both effective and safe for consumption, whether used as an antacid or a laxative.
What are the key elements involved in the calculation of the molar mass of MgOH2?
+The key elements involved in the calculation are the atomic masses of magnesium (Mg), hydrogen (H), and oxygen (O), and the number of atoms of each element present in the compound MgOH2.



