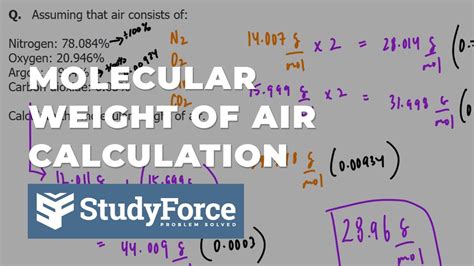
The molecular weight of air is a fundamental concept in various fields, including chemistry, physics, and engineering. Air is a mixture of gases, primarily consisting of nitrogen (N2) and oxygen (O2), with trace amounts of other gases such as argon (Ar), carbon dioxide (CO2), and water vapor (H2O). To calculate the molecular weight of air, we must consider the average molecular weight of its constituent gases and their respective proportions.
The molecular weight of a gas is the sum of the atomic weights of its constituent atoms. For example, the molecular weight of nitrogen (N2) is approximately 28.01 g/mol, which is the sum of the atomic weights of two nitrogen atoms (14.01 g/mol each). Similarly, the molecular weight of oxygen (O2) is approximately 32.00 g/mol, which is the sum of the atomic weights of two oxygen atoms (16.00 g/mol each).
Key Points
- The molecular weight of air is approximately 28.97 g/mol, which is a weighted average of the molecular weights of its constituent gases.
- The proportion of nitrogen (N2) in air is approximately 78.08%, while the proportion of oxygen (O2) is approximately 20.95%.
- Trace amounts of other gases, such as argon (Ar), carbon dioxide (CO2), and water vapor (H2O), also contribute to the molecular weight of air.
- The molecular weight of air can vary slightly depending on factors such as temperature, humidity, and altitude.
- Understanding the molecular weight of air is crucial in various applications, including aerospace engineering, atmospheric science, and chemical engineering.
Calculation of Molecular Weight of Air

To calculate the molecular weight of air, we can use the following formula:
Molecular weight of air = (Proportion of N2 x Molecular weight of N2) + (Proportion of O2 x Molecular weight of O2) + (Proportion of Ar x Molecular weight of Ar) +...
Using the approximate proportions of the constituent gases in air, we can calculate the molecular weight of air as follows:
Molecular weight of air = (0.7808 x 28.01 g/mol) + (0.2095 x 32.00 g/mol) + (0.0093 x 39.95 g/mol) +... ≈ 28.97 g/mol
Factors Affecting Molecular Weight of Air
The molecular weight of air can vary slightly depending on factors such as temperature, humidity, and altitude. For example, at higher temperatures, the proportion of water vapor in air increases, which can affect the molecular weight of air. Similarly, at higher altitudes, the proportion of oxygen in air decreases, which can also affect the molecular weight of air.
| Factor | Effect on Molecular Weight of Air |
|---|---|
| Temperature | Increases with increasing temperature due to increased water vapor content |
| Humidity | Increases with increasing humidity due to increased water vapor content |
| Altitude | Decreases with increasing altitude due to decreased oxygen content |

Applications of Molecular Weight of Air

The molecular weight of air has numerous applications in various fields, including:
Aerospace Engineering: The molecular weight of air is crucial in calculating the lift and drag forces on aircraft and spacecraft.
Atmospheric Science: The molecular weight of air is essential in understanding the behavior of the atmosphere and predicting weather patterns.
Chemical Engineering: The molecular weight of air is important in designing and optimizing chemical processes, such as gas separation and purification.
Future Directions
As our understanding of the molecular weight of air and its applications continues to evolve, we can expect new and innovative technologies to emerge. For example, advances in materials science and nanotechnology may lead to the development of more efficient gas separation membranes and sensors.
What is the molecular weight of air at sea level?
+The molecular weight of air at sea level is approximately 28.97 g/mol.
How does the molecular weight of air vary with altitude?
+The molecular weight of air decreases with increasing altitude due to the decrease in oxygen content.
What are the applications of molecular weight of air in aerospace engineering?
+The molecular weight of air is crucial in calculating the lift and drag forces on aircraft and spacecraft.
In conclusion, the molecular weight of air is a fundamental concept with numerous applications in various fields. Understanding the factors that affect the molecular weight of air and its applications is essential for accurate calculations and simulations. As our understanding of the molecular weight of air continues to evolve, we can expect new and innovative technologies to emerge.