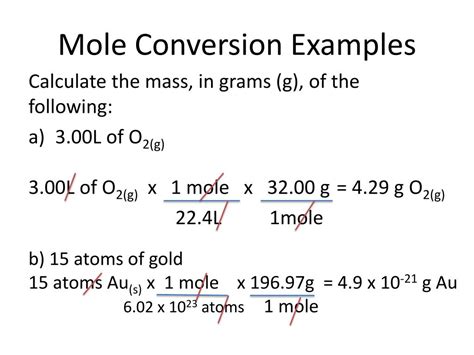
The concept of converting moles to atoms is fundamental in chemistry, as it allows scientists to understand the relationship between the amount of a substance and the number of particles it contains. A mole, defined as 6.022 x 10^23 particles (atoms or molecules), is a unit of measurement that facilitates calculations in chemical reactions and stoichiometry. In this article, we will explore five ways to approach the conversion from moles to atoms, emphasizing the importance of Avogadro's number and the practical applications of this conversion in various chemical contexts.
Key Points
- Understanding Avogadro's number (6.022 x 10^23 particles) is crucial for converting moles to atoms.
- The formula to convert moles to atoms involves multiplying the number of moles by Avogadro's number.
- Practical applications of mole-to-atom conversions are seen in chemical reactions, pharmaceuticals, and materials science.
- Calculations involving moles to atoms are essential for determining the yield of chemical reactions.
- Avogadro's hypothesis and the mole concept are foundational to modern chemistry, enabling precise quantitative measurements.
Introduction to Moles and Atoms
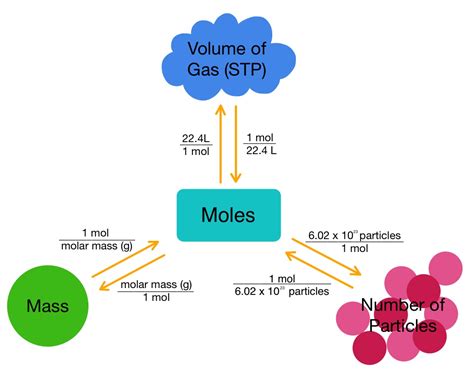
In chemistry, a mole (mol) is the unit of measurement in the International System of Units (SI) that represents 6.022 x 10^23 particles, which can be atoms, molecules, ions, or electrons. This number, known as Avogadro’s number, is named after the Italian scientist Amedeo Avogadro, who first proposed that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules. The mole is a critical concept in chemistry because it allows chemists to calculate the amounts of substances involved in chemical reactions, making it possible to predict the outcomes of reactions and the properties of the substances involved.
Understanding Avogadro’s Number
Avogadro’s number (6.022 x 10^23 particles) is a fundamental constant in chemistry that relates the amount of a substance to the number of particles it contains. This number is crucial for converting between moles and atoms because it represents the number of particles in one mole of any substance. By understanding and applying Avogadro’s number, chemists can convert the amount of a substance from moles to atoms or vice versa, facilitating calculations in chemical reactions and stoichiometry.
Converting Moles to Atoms
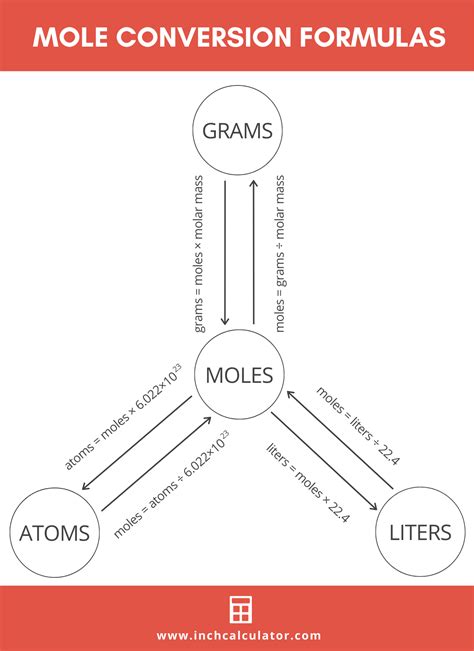
To convert moles to atoms, one must use the formula: number of atoms = number of moles * Avogadro’s number. This formula is straightforward and allows for the direct conversion from moles to atoms, provided Avogadro’s number is known. For example, if one has 2 moles of carbon atoms, the number of carbon atoms can be calculated as 2 moles * 6.022 x 10^23 atoms/mole = 1.2044 x 10^24 atoms. This calculation demonstrates how the mole concept, combined with Avogadro’s number, enables the conversion from the macroscopic scale (moles) to the microscopic scale (atoms).
Practical Applications
The conversion from moles to atoms has numerous practical applications in chemistry and related fields. In chemical synthesis, understanding the number of atoms involved in a reaction is crucial for determining the stoichiometry of the reaction and predicting the yield of the desired product. In pharmaceuticals, the precise calculation of drug molecules is essential for ensuring the efficacy and safety of medications. In materials science, the conversion from moles to atoms is used to understand the properties of materials at the atomic level, which is vital for the development of new materials with specific properties.
| Application Area | Importance of Moles to Atoms Conversion |
|---|---|
| Chemical Reactions | Determining reaction stoichiometry and yield |
| Pharmaceuticals | Ensuring drug efficacy and safety through precise molecular calculations |
| Materials Science | Understanding material properties at the atomic level for new material development |

Calculations and Examples
Calculations involving the conversion from moles to atoms are common in chemistry. For instance, if a chemist wants to know how many atoms are in 0.5 moles of oxygen gas (O2), they would first calculate the number of molecules of O2 and then the number of oxygen atoms. Since one molecule of O2 contains 2 oxygen atoms, the calculation would be: 0.5 moles * 6.022 x 10^23 molecules/mole * 2 atoms/molecule = 6.044 x 10^23 atoms. This example illustrates how the mole concept and Avogadro’s number are used to bridge the gap between the macroscopic and microscopic worlds in chemistry.
Challenges and Considerations
While the conversion from moles to atoms is straightforward using Avogadro’s number, challenges can arise in certain contexts, such as dealing with very small or very large quantities of substances. In such cases, precise calculations are critical, and any errors can significantly affect the outcome of chemical reactions or the properties of materials. Additionally, the conversion assumes ideal conditions and does not account for factors like the purity of the substance or environmental conditions that can affect chemical reactions.
What is Avogadro's number, and why is it important?
+Avogadro's number is 6.022 x 10^23 particles and is important because it allows for the conversion between the amount of a substance (in moles) and the number of particles (atoms or molecules) it contains.
How do chemists use the mole concept in practical applications?
+Chemists use the mole concept to calculate the amounts of substances involved in chemical reactions, predict the outcomes of reactions, and understand the properties of substances at the atomic level.
What are some challenges in converting moles to atoms?
+Challenges can include dealing with very small or large quantities, ensuring the purity of the substance, and accounting for environmental factors that can affect chemical reactions.
In conclusion, the conversion from moles to atoms is a fundamental concept in chemistry that relies on Avogadro’s number. Understanding this conversion is essential for various applications, from chemical synthesis and pharmaceuticals to materials science. By mastering the mole concept and Avogadro’s number, chemists can make precise calculations and predictions, contributing to advancements in chemistry and related fields. The importance of this concept underscores the need for a deep understanding of chemical principles and their practical applications, highlighting the intricate relationship between the macroscopic and microscopic worlds in chemistry.