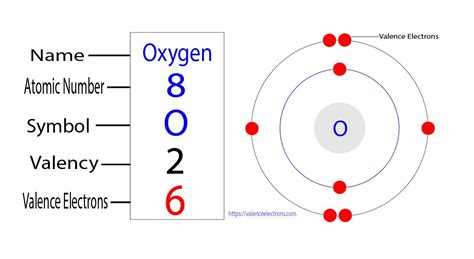
Oxygen, with its atomic number of 8, is a fundamental element in the periodic table, playing a crucial role in various biological and chemical processes. One of the key aspects of oxygen's chemical properties is its valence electrons, which determine how it interacts with other elements. In this article, we will delve into the world of oxygen valence electrons, exploring their significance, configuration, and the role they play in forming compounds.
Key Points
- Oxygen has 6 valence electrons, which are located in its outermost energy level.
- The valence electrons of oxygen are arranged in a 2s22p4 configuration.
- Oxygen tends to form compounds by gaining 2 electrons to achieve a full outer energy level, similar to the noble gas neon.
- The valence electrons of oxygen are crucial in determining its reactivity and the types of bonds it forms with other elements.
- Oxygen's valence electrons play a vital role in biological processes, such as cellular respiration and photosynthesis.
Oxygen Valence Electron Configuration
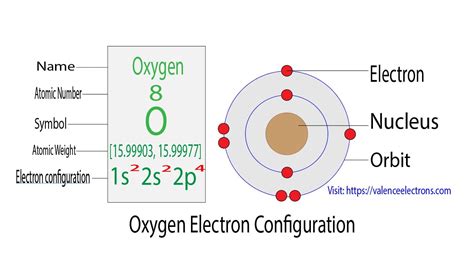
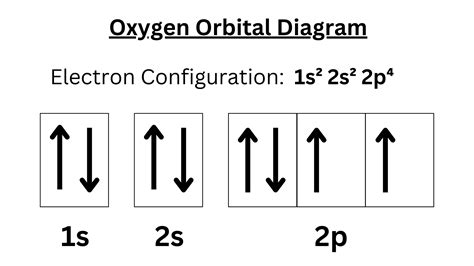
To understand the valence electrons of oxygen, it’s essential to look at its electron configuration. The electron configuration of oxygen is 1s22s22p4. The outermost energy level, which contains the valence electrons, is the second energy level (n = 2). This level has two subshells: 2s and 2p. The 2s subshell is completely filled with 2 electrons, while the 2p subshell has 4 electrons. These 6 electrons in the outermost energy level are the valence electrons of oxygen.
Significance of Oxygen Valence Electrons
The valence electrons of oxygen are significant because they determine how oxygen interacts with other elements. Oxygen’s tendency to gain 2 electrons to achieve a full outer energy level, similar to the noble gas neon, is a driving force behind its reactivity. This is why oxygen often forms compounds with metals and nonmetals, where it gains or shares electrons to achieve a stable electronic configuration. The valence electrons of oxygen also play a crucial role in determining the types of bonds it forms with other elements, such as covalent, ionic, or metallic bonds.
| Atomic Orbital | Electron Configuration |
|---|---|
| 1s | 2 electrons |
| 2s | 2 electrons |
| 2p | 4 electrons |
Role of Oxygen Valence Electrons in Biological Processes
Oxygen’s valence electrons play a crucial role in various biological processes, including cellular respiration and photosynthesis. In cellular respiration, oxygen acts as the final electron acceptor, allowing the transfer of electrons from glucose to oxygen, resulting in the production of energy in the form of ATP. In photosynthesis, oxygen is produced as a byproduct of the light-dependent reactions, where water is split to produce oxygen, protons, and electrons. The valence electrons of oxygen are essential for these processes, as they allow oxygen to interact with other molecules and facilitate the transfer of electrons.
Conclusion and Future Implications
In conclusion, the valence electrons of oxygen are a fundamental aspect of its chemical properties and play a vital role in determining its reactivity and the types of bonds it forms with other elements. Understanding the valence electrons of oxygen is essential for understanding various biological processes, such as cellular respiration and photosynthesis. As research continues to uncover the intricacies of oxygen’s valence electrons, we can expect to gain a deeper understanding of the complex interactions between oxygen and other elements, leading to new discoveries and applications in fields such as chemistry, biology, and medicine.
What is the electron configuration of oxygen?
+The electron configuration of oxygen is 1s22s22p4.
How many valence electrons does oxygen have?
+Oxygen has 6 valence electrons, which are located in its outermost energy level.
What is the significance of oxygen’s valence electrons in biological processes?
+Oxygen’s valence electrons play a crucial role in various biological processes, including cellular respiration and photosynthesis, by facilitating the transfer of electrons and allowing oxygen to interact with other molecules.