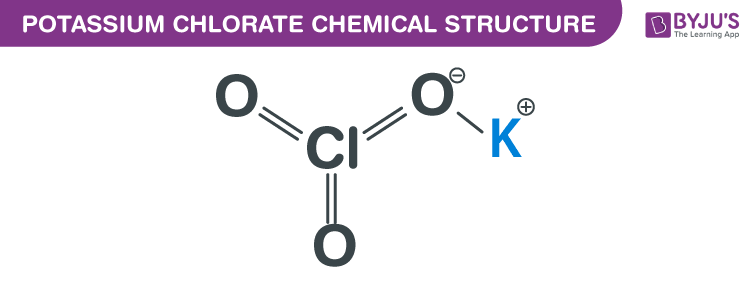
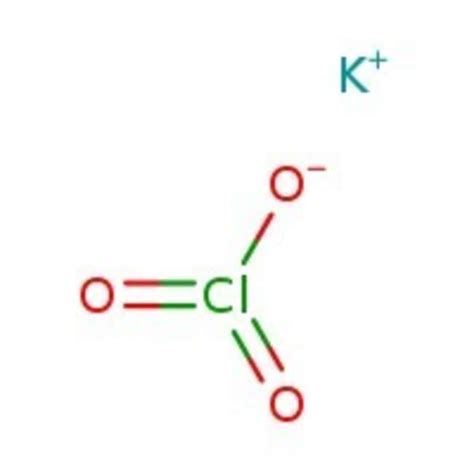
The potassium chlorate formula is a chemical compound that has been widely used in various applications, including matches, fireworks, and as a disinfectant. To understand the properties and uses of potassium chlorate, it is essential to start with its chemical formula, which is KClO3. This formula indicates that one molecule of potassium chlorate consists of one potassium (K) atom, one chlorine (Cl) atom, and three oxygen (O) atoms.
Chemical Structure and Properties
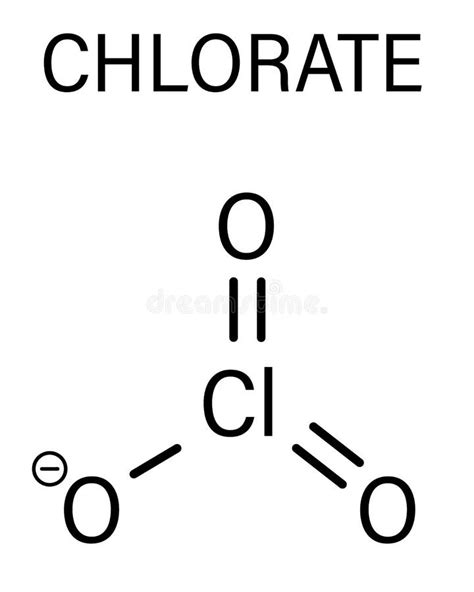
Potassium chlorate has a molecular weight of 122.55 g/mol and appears as a white or colorless crystalline powder. It is highly soluble in water and has a melting point of 400°C, although it decomposes before reaching its boiling point. The chemical structure of potassium chlorate can be represented as K+ClO3-, where the potassium ion (K+) is bonded to the chlorate ion (ClO3-). This ionic bonding is responsible for the compound’s high solubility in water and its reactivity with other substances.
Uses and Applications
Potassium chlorate has been used in various applications due to its strong oxidizing properties. One of its primary uses is in the production of matches, where it serves as an oxidizer to facilitate the combustion of the matchstick. It is also used in the manufacture of fireworks, where its oxidizing properties help to produce the colorful displays. Additionally, potassium chlorate has been used as a disinfectant due to its ability to release oxygen, which helps to kill bacteria and other microorganisms.
| Property | Value |
|---|---|
| Molecular Weight | 122.55 g/mol |
| Melting Point | 400°C |
| Boiling Point | Decomposes before boiling |
| Solubility in Water | Highly soluble |

Key Points
- The potassium chlorate formula is KClO3, indicating one potassium atom, one chlorine atom, and three oxygen atoms.
- Potassium chlorate is highly soluble in water and has a melting point of 400°C.
- The compound is used in the production of matches, fireworks, and as a disinfectant due to its strong oxidizing properties.
- Potassium chlorate has a molecular weight of 122.55 g/mol and appears as a white or colorless crystalline powder.
- Handling potassium chlorate requires care due to its potential to decompose and release oxygen, which can be hazardous.
Safety Precautions and Handling
When handling potassium chlorate, it is essential to take necessary safety precautions to avoid accidents. The compound should be stored in a cool, dry place away from flammable materials and sources of heat. It is also crucial to wear protective gear, including gloves and safety glasses, when handling potassium chlorate to prevent skin and eye irritation. In case of accidental ingestion or exposure, it is vital to seek medical attention immediately.
Environmental Impact
The production and use of potassium chlorate can have environmental implications. The compound can contaminate water sources and soil if not disposed of properly. Additionally, the release of oxygen during decomposition can contribute to the formation of ground-level ozone, a component of smog. Therefore, it is essential to follow proper disposal procedures and regulations to minimize the environmental impact of potassium chlorate.
What are the primary uses of potassium chlorate?
+Potassium chlorate is primarily used in the production of matches, fireworks, and as a disinfectant due to its strong oxidizing properties.
What are the safety precautions when handling potassium chlorate?
+When handling potassium chlorate, it is essential to store it in a cool, dry place, wear protective gear, and avoid sources of heat to prevent accidents.
What is the environmental impact of potassium chlorate?
+The production and use of potassium chlorate can contaminate water sources and soil, and contribute to the formation of ground-level ozone if not disposed of properly.
In conclusion, potassium chlorate is a versatile compound with various applications due to its strong oxidizing properties. Understanding its chemical structure, properties, and uses is essential for handling and utilizing the compound safely and effectively. By following proper safety precautions and disposal procedures, we can minimize the environmental impact of potassium chlorate and ensure its continued use in various industries.