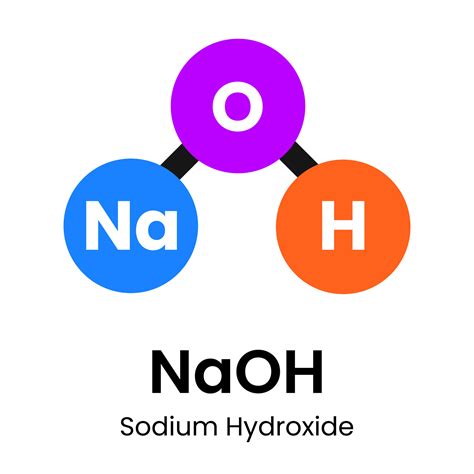
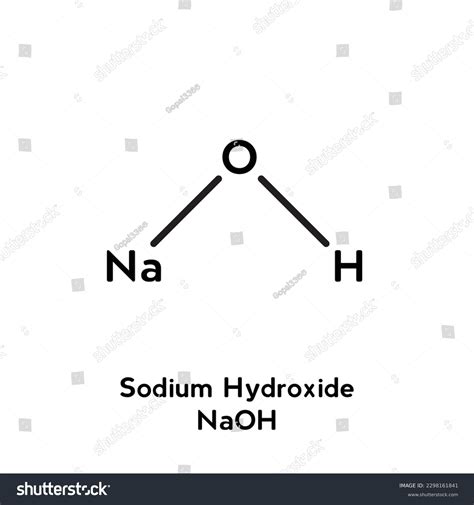
Sodium hydroxide, commonly known as lye or caustic soda, is a highly alkaline substance with a wide range of industrial, commercial, and household applications. Its chemical formula is NaOH, indicating that it is composed of one sodium (Na) atom, one oxygen (O) atom, and one hydrogen (H) atom. This compound is highly soluble in water and is known for its strong corrosive properties, making it a critical component in various manufacturing processes, including the production of paper, textiles, and soap.
Key Points
- The chemical formula of sodium hydroxide is NaOH, representing its composition of sodium, oxygen, and hydrogen.
- Sodium hydroxide is highly soluble in water, creating a strong alkaline solution.
- It has extensive applications in industries such as paper, textiles, and soap manufacturing due to its corrosive properties.
- The handling of sodium hydroxide requires caution due to its potential to cause severe burns and eye damage.
- Sodium hydroxide plays a crucial role in various chemical reactions, serving as a strong base.
Properties and Applications of Sodium Hydroxide
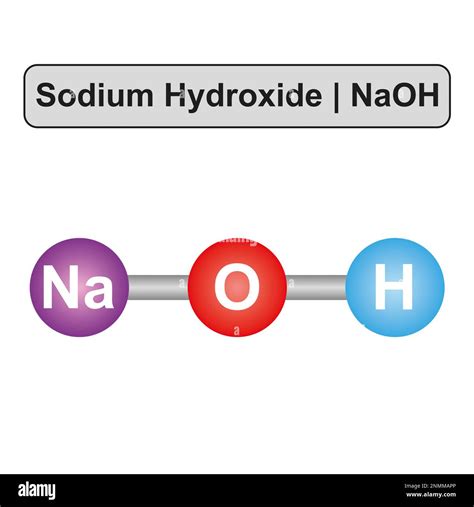
Sodium hydroxide is characterized by its white, odorless solid appearance and its ability to absorb moisture from the air, leading to its deliquescent nature. When dissolved in water, it releases heat, forming a highly alkaline solution. This exothermic reaction, along with its high solubility, contributes to its effectiveness in various applications. For instance, in the paper industry, sodium hydroxide is used in the pulping process to break down lignin, a component of wood, facilitating the production of paper products. Similarly, in the textile industry, it is utilized for the mercerization of cotton, which improves the fiber’s strength and dyeability.
Chemical Reactions Involving Sodium Hydroxide
Sodium hydroxide is a strong base that readily reacts with acids to form salts and water. This property makes it a versatile reagent in chemical synthesis. For example, the reaction between sodium hydroxide and hydrochloric acid (HCl) yields sodium chloride (NaCl, or common table salt) and water, as represented by the equation: NaOH + HCl → NaCl + H2O. Such reactions are fundamental in the production of various chemicals and in neutralizing acidic substances in industrial processes.
| Chemical Reaction | Products |
|---|---|
| NaOH + HCl | NaCl + H2O |
| NaOH + CO2 | NaHCO3 |
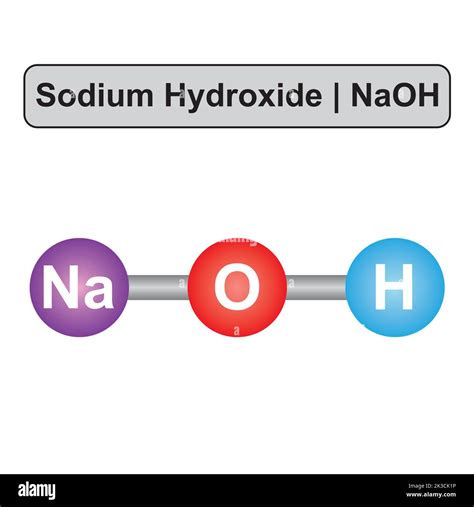
Safety and Handling of Sodium Hydroxide
Due to its highly corrosive nature, sodium hydroxide poses significant risks to human health and safety. Direct contact with the skin can cause severe burns, and eye exposure can lead to blindness. Inhalation of sodium hydroxide dust or fumes can irritate the respiratory system. Therefore, handling sodium hydroxide requires the use of protective equipment, including gloves, goggles, and a face mask. It is also crucial to follow proper procedures for storage and disposal to prevent accidents and environmental contamination.
Environmental and Health Implications
The production and use of sodium hydroxide can have environmental implications, primarily related to the release of toxic substances during manufacturing and the potential for water pollution. Furthermore, the improper disposal of sodium hydroxide can contaminate soil and water sources, posing risks to both human health and wildlife. Regulatory measures and industrial practices aimed at minimizing waste and preventing environmental contamination are essential for mitigating these risks.
What is the chemical formula of sodium hydroxide?
+The chemical formula of sodium hydroxide is NaOH, indicating it is composed of sodium, oxygen, and hydrogen.
What are the primary applications of sodium hydroxide?
+Sodium hydroxide is used in the manufacturing of paper, textiles, and soap, as well as in various chemical reactions due to its strong alkaline properties.
What precautions should be taken when handling sodium hydroxide?
+Handling sodium hydroxide requires protective equipment, including gloves, goggles, and a face mask, to prevent skin contact, eye damage, and inhalation of dust or fumes.
In conclusion, sodium hydroxide, with its chemical formula NaOH, plays a critical role in various industrial and chemical processes. Its properties as a strong base and its reactivity with acids make it a versatile compound in manufacturing. However, its handling and use require caution and adherence to safety protocols due to its corrosive nature. Understanding the chemical, environmental, and safety aspects of sodium hydroxide is essential for its effective and responsible application across different sectors.