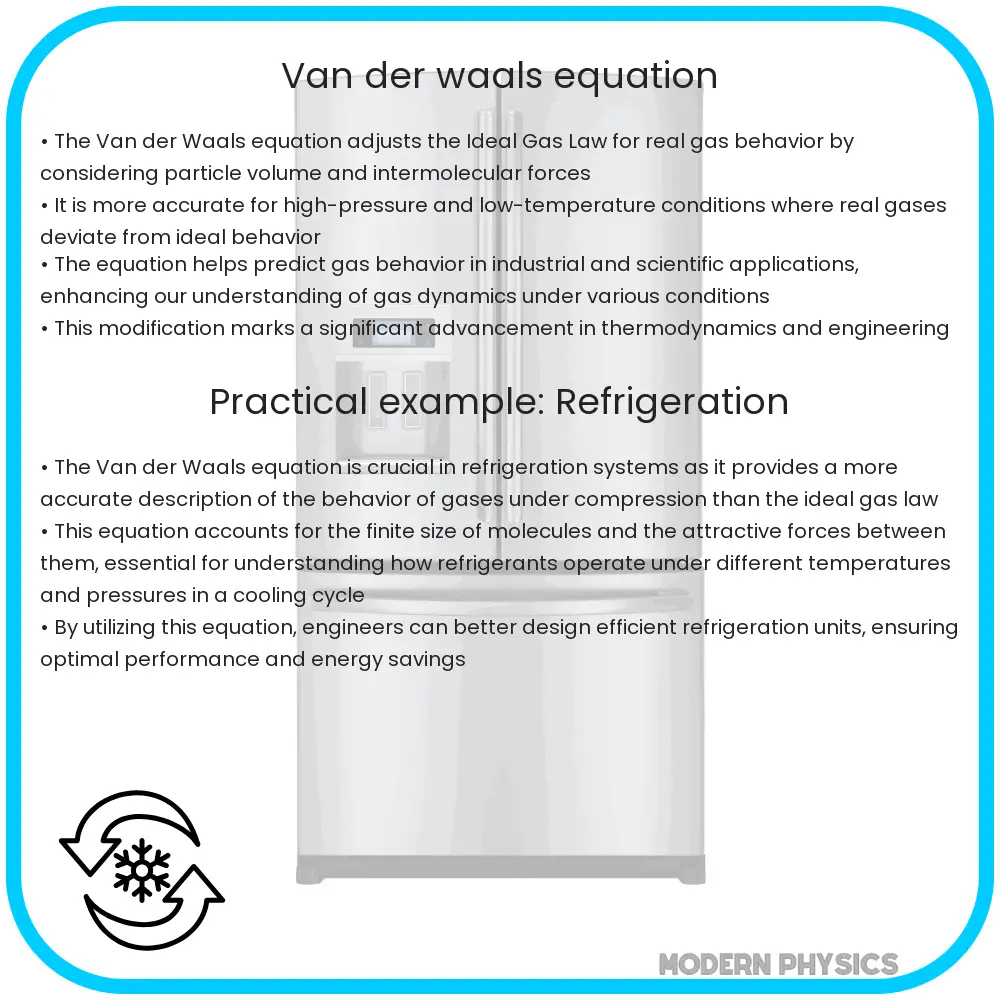
Understanding the intricacies of Van der Waals forces is crucial in various fields of science and engineering, including chemistry, physics, and materials science. These forces, named after the Dutch scientist Johannes Diderik van der Waals, are weak intermolecular forces that arise due to temporary dipoles formed in atoms or molecules. They play a significant role in determining the physical properties of substances, such as melting and boiling points, solubility, and surface tension. Here, we'll delve into five key tips related to Van der Waals forces, exploring their nature, significance, and applications.
Tip 1: Understanding the Nature of Van der Waals Forces
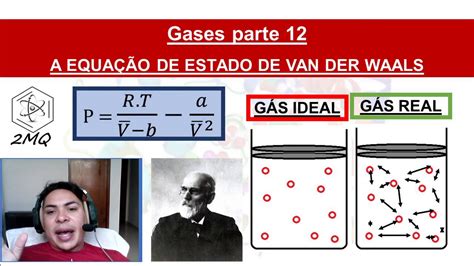
Van der Waals forces are classified into three main types: London dispersion forces, dipole-dipole forces, and dipole-induced dipole forces. London dispersion forces are the weakest and are present in all molecules, regardless of their polarity. They arise due to the temporary dipoles formed by the movement of electrons around atoms. Dipole-dipole forces occur between two polar molecules, while dipole-induced dipole forces are between a polar molecule and a non-polar molecule. Understanding the type of Van der Waals force involved is essential for predicting the behavior of substances.
Importance of Molecular Shape and Size
The shape and size of molecules significantly influence the strength of Van der Waals forces. Larger molecules tend to have stronger London dispersion forces due to their larger electron clouds, which can temporary distort and form dipoles more easily. The shape of a molecule can also affect how closely molecules can approach each other, thereby influencing the strength of these forces. For instance, branched molecules may have weaker Van der Waals forces compared to straight-chain molecules due to less efficient packing.
| Type of Force | Description | Strength |
|---|---|---|
| London Dispersion Forces | Present in all molecules | Weakest |
| Dipole-Dipole Forces | Between two polar molecules | Stronger than London |
| Dipole-Induced Dipole Forces | Between polar and non-polar molecules | Variable |
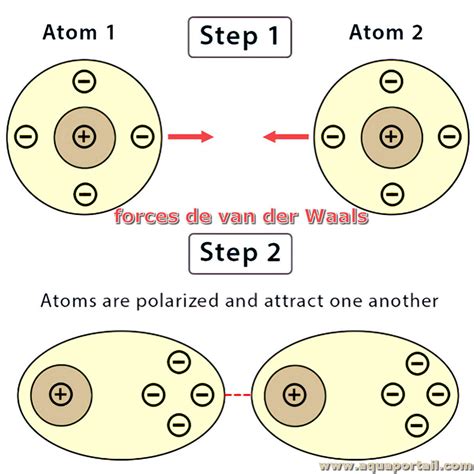
Tip 2: Role in Phase Transitions
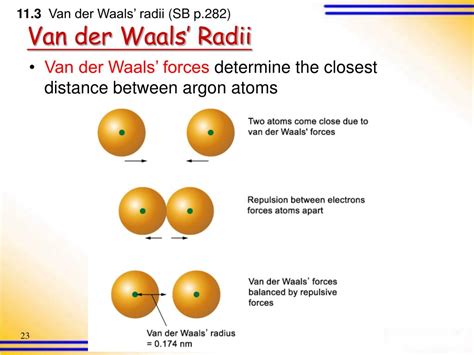
Van der Waals forces play a pivotal role in phase transitions, such as melting and boiling. The strength of these forces between molecules influences the energy required to separate molecules and change their state. Substances with stronger Van der Waals forces tend to have higher melting and boiling points because more energy is needed to overcome these forces and transition from solid to liquid (melting) or from liquid to gas (boiling).
Effect on Surface Tension
Surface tension, the property of the surface of a liquid that allows it to resist an external force due to the cohesive nature of its molecules, is also influenced by Van der Waals forces. In liquids, these forces act between molecules at the surface and those below it, contributing to the phenomenon of surface tension. Understanding this relationship is vital for applications involving liquid surfaces, such as in the study of surfactants or in biomedical devices that interact with bodily fluids.
Key Points
- Van der Waals forces are crucial in understanding the physical properties of substances.
- The strength of these forces influences melting and boiling points.
- Molecular shape and size significantly affect the strength of Van der Waals forces.
- These forces contribute to the surface tension of liquids.
- Understanding Van der Waals forces is essential for designing materials with specific properties.
Tip 3: Application in Materials Science
In materials science, the manipulation of Van der Waals forces is a key strategy for developing materials with tailored properties. For example, nanomaterials, which have unique properties due to their small size, can exhibit altered Van der Waals interactions due to their high surface-to-volume ratio. This can lead to novel applications in fields such as energy storage, catalysis, and drug delivery.
Nanotechnology and Van der Waals Forces
The design of nanostructures often involves the consideration of Van der Waals forces to achieve specific functionalities. For instance, in the development of graphene, a material with remarkable mechanical and electrical properties, the Van der Waals interactions between graphene layers are crucial for its stability and performance. Similarly, in the fabrication of nanocomposites, the control of Van der Waals forces between different components can enhance the material’s strength, conductivity, or optical properties.
| Application | Description | Impact of Van der Waals Forces |
|---|---|---|
| Nanomaterials | High surface-to-volume ratio materials | Altered interactions due to size |
| Graphene | Layered carbon material | Interlayer forces crucial for stability |
| Nanocomposites | Materials with nano-scale components | Control of forces enhances properties |
Tip 4: Biological Relevance
Van der Waals forces also play a significant role in biological systems. They are involved in the interactions between molecules such as proteins, DNA, and cell membranes. The specificity and strength of these interactions can influence biological processes, including protein folding, enzyme-substrate binding, and cell adhesion. Understanding these forces is essential for developing drugs that can effectively bind to target molecules and for designing biomaterials that can interact favorably with living tissues.
Protein-Ligand Interactions
The binding of ligands to proteins is a critical process in biochemistry, influenced by Van der Waals forces among other types of interactions. The shape and chemical properties of the binding site on the protein, as well as the molecular properties of the ligand, determine the strength and specificity of the interaction. This understanding is fundamental for drug design, where the goal is to create molecules that can bind selectively and with high affinity to specific protein targets, thereby modulating their activity.
What are the main types of Van der Waals forces?
+The main types of Van der Waals forces are London dispersion forces, dipole-dipole forces, and dipole-induced dipole forces.
How do Van der Waals forces affect the melting and boiling points of substances?
+Substances with stronger Van der Waals forces tend to have higher melting and boiling points because more energy is required to overcome these forces and change the state of the substance.
What is the role of Van der Waals forces in biological systems?
+Van der Waals forces are involved in the interactions between biomolecules such as proteins, DNA, and cell membranes, influencing processes like protein folding, enzyme-substrate binding, and cell adhesion.
Tip 5: Future Directions and Challenges
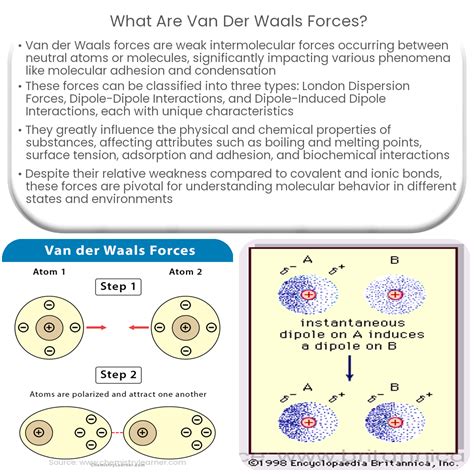
As research continues to uncover the intricacies of Van der Waals forces, new challenges and opportunities emerge. The development of computational models that can accurately predict the behavior of these forces at various scales, from molecular to macroscopic, is a significant challenge. Additionally, the integration of Van der Waals forces into the design of novel materials and biological systems requires a deep understanding of their role in determining material properties and biological functions.
Advances in Computational Modeling
Recent advances in computational power and modeling techniques have enabled more accurate simulations of Van der Waals forces. Methods such as density functional theory (DFT) and molecular dynamics simulations are being refined to better capture the nuances of these interactions. These advancements hold promise for predicting the properties of materials and biological systems with greater precision, thereby accelerating discovery and innovation.
In conclusion, Van der Waals forces are a fundamental aspect of intermolecular interactions, playing a critical role in determining the properties of substances and their behavior in various contexts. From materials science to biological systems, understanding and manipulating these forces is key to advancing our knowledge and capabilities. As research in this area continues to evolve, we can expect significant breakthroughs in fields such as energy, medicine, and technology, driven by our deepening understanding of the intricate world of Van der Waals forces.