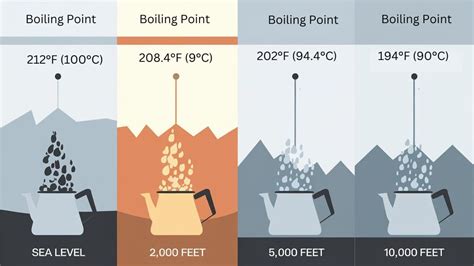
When discussing the fundamental principles of thermodynamics, one of the most universally recognized concepts is the boiling point of water. At standard atmospheric pressure, water boils at 212 degrees Fahrenheit (°F) or 100 degrees Celsius (°C). This specific temperature is a critical benchmark in various scientific, culinary, and industrial applications. Understanding the boiling point of water and its implications is essential for a wide range of activities, from cooking and brewing to chemical engineering and environmental science.
Key Points
- The boiling point of water at standard atmospheric pressure is 212°F or 100°C.
- Varying atmospheric pressures can alter the boiling point of water.
- Understanding the boiling point is crucial for applications in cooking, chemistry, and engineering.
- The boiling point of water is a fundamental constant in scientific research and education.
- Different substances have unique boiling points, which are critical in chemical and physical analyses.
The Science Behind the Boiling Point of Water

The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, allowing bubbles to form and the liquid to change state to a gas. For water, this process occurs at 212°F (100°C) under standard conditions. However, it’s important to note that the boiling point can be affected by changes in atmospheric pressure. At higher elevations, where atmospheric pressure is lower, water will boil at a lower temperature. Conversely, at greater depths or in environments with higher pressures, water will boil at a higher temperature.
Factors Influencing the Boiling Point of Water
Besides atmospheric pressure, other factors can influence the boiling point of water, including the presence of impurities or the application of external pressures. For example, dissolved solids in water can raise its boiling point, a phenomenon known as boiling-point elevation. This principle is utilized in various industrial and culinary processes to control the boiling point of solutions. Additionally, the shape and material of the container can affect the boiling process due to variations in heat transfer efficiency.
| Factor | Effect on Boiling Point |
|---|---|
| Atmospheric Pressure Increase | Boiling point increases |
| Atmospheric Pressure Decrease | Boiling point decreases |
| Dissolved Solids | Boiling point increases |
| Purities and Impurities | Can decrease or increase boiling point depending on the nature of the impurity |
Applications of Boiling Point in Various Fields

The boiling point of water has numerous practical applications across different fields. In cooking, understanding the boiling point is essential for preparing meals safely and efficiently. In chemistry and chemical engineering, knowing the boiling points of various substances, including water, is critical for separation processes, purification, and the synthesis of new compounds. In environmental science, the boiling point of water plays a role in understanding climate patterns, the water cycle, and the behavior of aqueous solutions in natural systems.
Importance in Daily Life and Industrial Processes
Beyond scientific applications, the boiling point of water impacts daily life in several ways. For instance, the efficiency of household appliances like kettles and boilers can be influenced by the boiling point of water. In industrial processes, such as distillation and steam generation, precise control over the boiling point of water is necessary for operational efficiency and safety. Furthermore, the boiling point of water is a basic yet vital piece of knowledge in first aid and survival skills, where it can be crucial for sterilizing water and cooking food safely in emergency situations.
What is the significance of the boiling point of water in cooking?
+The boiling point of water is significant in cooking as it affects the texture, taste, and safety of food. Proper boiling ensures that food is cooked evenly and that harmful bacteria are killed, making the food safe to eat.
How does atmospheric pressure affect the boiling point of water?
+Atmospheric pressure directly affects the boiling point of water. At higher altitudes where the atmospheric pressure is lower, water boils at a lower temperature. Conversely, at lower altitudes or in pressurized environments, water boils at a higher temperature.
What are some practical applications of understanding the boiling point of water?
+Understanding the boiling point of water has practical applications in cooking, chemistry, environmental science, and industrial processes. It's essential for food safety, chemical synthesis, and the efficient operation of steam-powered machinery.
In conclusion, the boiling point of water at 212 degrees Fahrenheit or 100 degrees Celsius is a fundamental constant with far-reaching implications across various disciplines. From the intricacies of thermodynamic principles to the practicalities of daily life and industrial operations, understanding the factors that influence the boiling point of water and its applications is invaluable. As science and technology continue to evolve, the significance of this basic yet profound concept will remain a cornerstone of human knowledge and innovation.