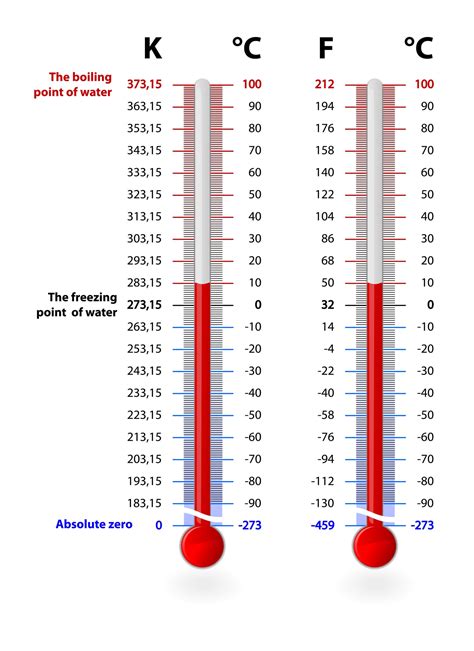
Freezing temperatures are a critical aspect of various scientific and engineering disciplines, including physics, chemistry, and materials science. The temperature at which a substance freezes is a fundamental physical constant that is essential for understanding the behavior of materials under different conditions. In this article, we will explore the concept of freezing temperatures, their significance, and the factors that influence them.
Key Points
- The freezing point of a substance is the temperature at which it changes state from a liquid to a solid.
- Freezing temperatures are affected by factors such as pressure, purity, and the presence of impurities.
- The freezing point of water is 0°C (32°F) at standard atmospheric pressure.
- Some substances, such as helium, do not freeze at atmospheric pressure, regardless of the temperature.
- Understanding freezing temperatures is crucial for various applications, including cryogenics, materials science, and environmental science.
Introduction to Freezing Temperatures

Freezing temperatures are a critical aspect of understanding the behavior of materials under different conditions. The freezing point of a substance is the temperature at which it changes state from a liquid to a solid. This temperature is a fundamental physical constant that is essential for understanding the properties and behavior of materials. In this section, we will explore the concept of freezing temperatures and their significance in various scientific and engineering disciplines.
Factors Influencing Freezing Temperatures
Freezing temperatures are influenced by several factors, including pressure, purity, and the presence of impurities. The freezing point of a substance can be affected by changes in pressure, with some substances freezing at higher temperatures under increased pressure. The presence of impurities can also affect the freezing point of a substance, with some impurities lowering the freezing point and others raising it. Understanding these factors is crucial for predicting and controlling the behavior of materials under different conditions.
| Substance | Freezing Point (°C) |
|---|---|
| Water | 0 |
| Helium | -269 (at 1 atm) |
| Nitrogen | -210 |
| Oxygen | -219 |
| Carbon Dioxide | -56.6 (at 1 atm) |
Applications of Freezing Temperatures

Freezing temperatures have a wide range of applications in various scientific and engineering disciplines, including cryogenics, materials science, and environmental science. In cryogenics, the freezing point of substances is used to achieve extremely low temperatures, which are essential for various applications, including superconductivity and superfluidity. In materials science, understanding the freezing point of substances is crucial for predicting and controlling the behavior of materials under different conditions. In environmental science, the freezing point of substances is used to study the behavior of materials in different environments, including the Earth’s atmosphere and oceans.
Cryogenics and Superconductivity
Cryogenics is the study of the behavior of materials at extremely low temperatures, typically below -150°C. At these temperatures, some materials exhibit unique properties, including superconductivity and superfluidity. Superconductivity is the ability of a material to conduct electricity with zero resistance, while superfluidity is the ability of a material to flow without viscosity. Understanding the freezing point of substances is crucial for achieving these extremely low temperatures and for predicting and controlling the behavior of materials under different conditions.
What is the freezing point of water at standard atmospheric pressure?
+The freezing point of water at standard atmospheric pressure is 0°C (32°F).
What factors influence the freezing point of a substance?
+The freezing point of a substance is influenced by several factors, including pressure, purity, and the presence of impurities.
What is the significance of freezing temperatures in materials science?
+Understanding the freezing point of substances is crucial for predicting and controlling the behavior of materials under different conditions, including the formation of crystals, the growth of materials, and the properties of materials at different temperatures.
In conclusion, freezing temperatures are a critical aspect of various scientific and engineering disciplines, including physics, chemistry, and materials science. Understanding the freezing point of substances is essential for predicting and controlling the behavior of materials under different conditions, including the formation of crystals, the growth of materials, and the properties of materials at different temperatures. By exploring the concept of freezing temperatures and their significance, we can gain a deeper understanding of the behavior of materials and develop new technologies and applications that rely on the unique properties of materials at extremely low temperatures.