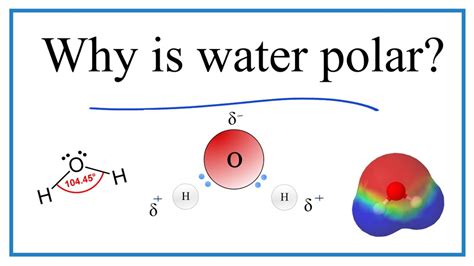
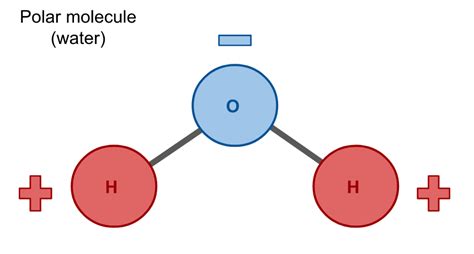
The molecular structure of water, commonly referred to as H2O, plays a crucial role in its polarity. Polarity in chemistry is a phenomenon where a molecule has a slight positive charge on one side and a slight negative charge on the other, resulting from the unequal sharing of electrons between atoms in a covalent bond. Water molecules are composed of two hydrogen atoms and one oxygen atom, with the oxygen atom being more electronegative than the hydrogen atoms. This means that oxygen has a stronger tendency to attract electrons, causing the shared electrons in the covalent bonds between oxygen and hydrogen to be pulled closer to the oxygen nucleus.
This uneven distribution of electrons leads to a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom, giving the water molecule a dipole moment. The dipole moment is a measure of the separation of positive and negative electrical charges within a molecule. It's this dipole moment that makes water a polar molecule, which is critical for its role in biological systems and chemical reactions. The polarity of water allows it to form hydrogen bonds with other water molecules and with other polar or ionic substances, which is essential for many of its unique properties and its ability to dissolve a wide variety of compounds.
Key Points
- The polarity of water arises from the difference in electronegativity between oxygen and hydrogen atoms in the molecule.
- The partial positive charge on hydrogen atoms and the partial negative charge on the oxygen atom create a dipole moment, characterizing water as a polar molecule.
- The polarity of water enables it to form hydrogen bonds, which are crucial for its solvent properties and its role in biological systems.
- The unique properties of water, such as its high surface tension and boiling point, are also influenced by its polarity and the hydrogen bonding between molecules.
- Understanding the polarity of water is essential for appreciating its chemical and biological significance, including its role in chemical reactions, biological processes, and environmental systems.
Electronegativity and Molecular Structure
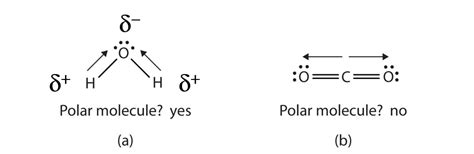
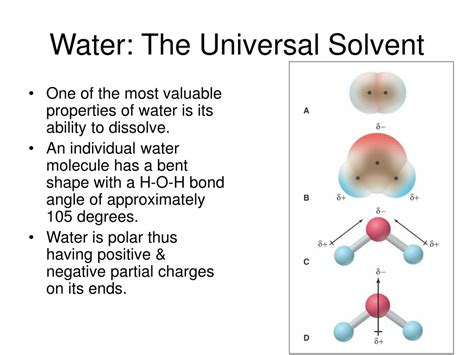
The concept of electronegativity is central to understanding the polarity of molecules like H2O. Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. Oxygen, with an electronegativity value of approximately 3.44 on the Pauling scale, is more electronegative than hydrogen, which has an electronegativity value of about 2.20. This difference in electronegativity leads to the unequal sharing of electrons in the O-H bonds of a water molecule, resulting in the oxygen atom having a slight negative charge (δ-) and each hydrogen atom having a slight positive charge (δ+).
Hydrogen Bonding and Solvent Properties
The polarity of water and the resulting ability to form hydrogen bonds are key factors in its solvent properties. Hydrogen bonds are relatively weak bonds that form between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another. These bonds are crucial for the dissolution of ionic and polar substances in water, as they allow water molecules to surround and interact with the charged particles of the substance, effectively dissolving them. The ability of water to dissolve a wide variety of compounds makes it an indispensable solvent in both biological systems and industrial applications.
| Property | Value |
|---|---|
| Electronegativity of Oxygen | 3.44 (Pauling scale) |
| Electronegativity of Hydrogen | 2.20 (Pauling scale) |
| Dipole Moment of Water | 1.85 Debye |
| Boiling Point of Water | 100°C at 1 atm |
Biological and Environmental Significance
The polarity of water has profound implications for its role in biological systems. Water is the medium in which many biochemical reactions occur, and its solvent properties allow it to facilitate the transport of nutrients, ions, and waste products across cell membranes. The polarity of water also influences the structure and function of biological molecules, such as proteins and nucleic acids, by forming hydrogen bonds that contribute to their three-dimensional conformation and stability. In environmental systems, the polarity of water affects the distribution and transport of substances, influencing processes such as weathering, erosion, and the cycling of nutrients.
Implications for Chemical Reactions
The polarity of water impacts its role as a solvent in chemical reactions. Polar substances are more soluble in water, which facilitates reactions involving these compounds. Water’s polarity also influences the rate and equilibrium of chemical reactions, as it can stabilize transition states or intermediates through hydrogen bonding, thus affecting the reaction kinetics. Moreover, the high dielectric constant of water, a result of its polarity, helps to reduce the electrostatic forces between charged particles, further facilitating the dissolution and reaction of ionic substances.
What makes water a polar molecule?
+Water is a polar molecule due to the difference in electronegativity between oxygen and hydrogen atoms, leading to an unequal sharing of electrons and the creation of a dipole moment.
Why is the polarity of water important for its solvent properties?
+The polarity of water allows it to form hydrogen bonds with other water molecules and with polar or ionic substances, which is essential for its ability to dissolve a wide variety of compounds.
How does the polarity of water influence biological systems?
+The polarity of water influences the structure and function of biological molecules and facilitates the transport of substances across cell membranes, playing a critical role in many biological processes.
In conclusion, the polarity of H2O, arising from the difference in electronegativity between its constituent atoms, is a fundamental property that underlies many of its unique characteristics and roles in chemical, biological, and environmental systems. Understanding the polarity of water provides insights into its solvent properties, its ability to form hydrogen bonds, and its significance in facilitating chemical reactions and biological processes. As such, the study of water’s polarity remains a vital area of scientific inquiry, with implications for fields ranging from chemistry and biology to ecology and environmental science.